产品介绍:
产品说明
一般描述
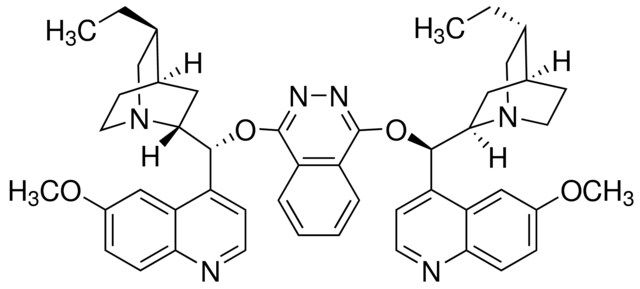
DHQ)2PHAL is a modified cinchona alkaloid.
应用
(DHQ)2PHAL may be used in the following processes:
- As a catalyst Asymmetric and chemoselective N-allylic alkylation of indoles with Morita-Baylis-Hillman carbonates to form pyrrolo[1,2-a]indole and pyrrolo[3,2,1-ij]quinoline derivatives.
- As a ligand for the osmium catalyzed-Sharpless asymmetric dihydroxylation step of (S)-a-benzoyloxy carboxylic acids multistep synthesis.
- As a ligand for the carbamate based asymmetric aminohydroxylation of styrene derivatives to form N-carbamate protected R-arylglycinols.
Sharpless 不对称双羟基化的配体,用于由 (E,E)- 或 (E,Z)-1,3-二烯酸甲酯不对称制备 syn-3,5-羟基羧酸酯。
包装
500 mg in glass bottle
外形
具有三取代 C=C 键的不饱和酯的不对称双羟基化。
法律信息
经 Shasun Chemicals and Drugs Limited 授权销售。
基本信息
| 经验(实验)分子式 | C48H54N6O4 |
| 分子量 | 778.98 |
| Beilstein | 5475677 |
| MDL编号 | MFCD00191975 |
| PubChem化学物质编号 | 24864480 |
| NACRES | NA.22 |
产品性质
| 质量水平 | 200 |
| 测定 | ≥95% |
| 旋光性 | [α]22/D +336°, c = 1.2 in methanol |
| mp | 178 ℃ (dec.) (lit.) |
| SMILES string | CC[C@@H]1CN2CCC1CC2[C@H](Oc3nnc(O[C@@H](C4CC5CCN4C[C@@H]5CC)c6ccnc7ccc(OC)cc67)c8ccccc38)c9ccnc%10ccc(OC)cc9%10 |
| InChI | 1S/C48H54N6O4/c1-5-29-27-53-21-17-31(29)23-43(53)45(35-15-19-49-41-13-11-33(55-3)25-39(35)41)57-47-37-9-7-8-10-38(37)48(52-51-47)58-46(44-24-32-18-22-54(44)28-30(32)6-2)36-16-20-50-42-14-12-34(56-4)26-40(36)42/h7-16,19-20,25-26,29-32,43-46H,5-6,17-18,21-24,27-28H2,1-4H3/t29-,30+,31-,32+,43-,44-,45-,46-/m1/s1 |
| InChI key | YUCBLVFHJWOYDN-PPIALRKJSA-N |
安全信息
| 象形图 |  |
| 警示用语: | Warning |
| 危险声明 | H317 |
| 预防措施声明 | P280 - P302 + P352 |
| 危险分类 | Skin Sens. 1 |
| 储存分类代码 | 11 - Combustible Solids |
| WGK | WGK 3 |
| 闪点(F) | Not applicable |
| 闪点(C) | Not applicable |
| 个人防护装备 | Eyeshields, Faceshields, Gloves, type N95 (US) |